Industry news
Three minutes to teach you to distinguish: sterilization, sterilization, disinfection
time:2020-12-04 view count:671
1. Sterilization
Treatment to kill or remove microorganisms on the media, including bacterial spores with strong resistance.
Note that sterilization is for microorganisms, including not only bacteria, but also spores, viruses, fungi, mycoplasma, chlamydia and so on.
The methods that fall into this category include: thermal sterilization (steam, dry heat), ultra-high pressure sterilization, ionizing radiation sterilization (≥25kGy), microwave sterilization, plasma sterilization and other physical sterilization methods, as well as the use of oxyacetic acid, A method of sterilization by chemical methods such as hydrogen peroxide.
Sterilization effect evaluation commonly used biological indicator bacteria: Bacillus Stearothermophilus (Bacillus Stearothermophilus ATCC 7953 or SSI K31) spores.
The amount of recovered bacteria is 5×105cfu/tablet~5×106cfu/tablet, or 5×105cfu/ml~5×106cfu/ml.
The survival probability (ie sterility assurance level, SAL) of microorganisms in terminally sterilized products shall not be higher than 10-6.
High temperature sterilized milk (UHT milk) is an instantaneous sterilization process of ultra-high temperature instantaneous sterilization (135°C to 150°C, 4 to 15 seconds) to completely destroy the microorganisms and spores that can grow in it.

2. Sterile
Sterility is the result of sterilization, meaning that it is completely free of microorganisms. The operation technique to prevent microorganisms from entering the body or object becomes aseptic operation.
However, due to the limitations of the current testing methods, the concept of sterility cannot be applied to the evaluation of the sterility of the entire batch of products. Therefore, the concept of "sterile" currently used is "sterile" in the sense of probability.
After terminal sterilization, the sterility assurance level of sterile products is that the probability of residual microbial contamination ≤ 10-6.
"Commercial sterility" refers to the state in which canned food undergoes moderate heat sterilization and does not contain pathogenic microorganisms or non-pathogenic microorganisms that can multiply in it at normal temperature (GB8950).
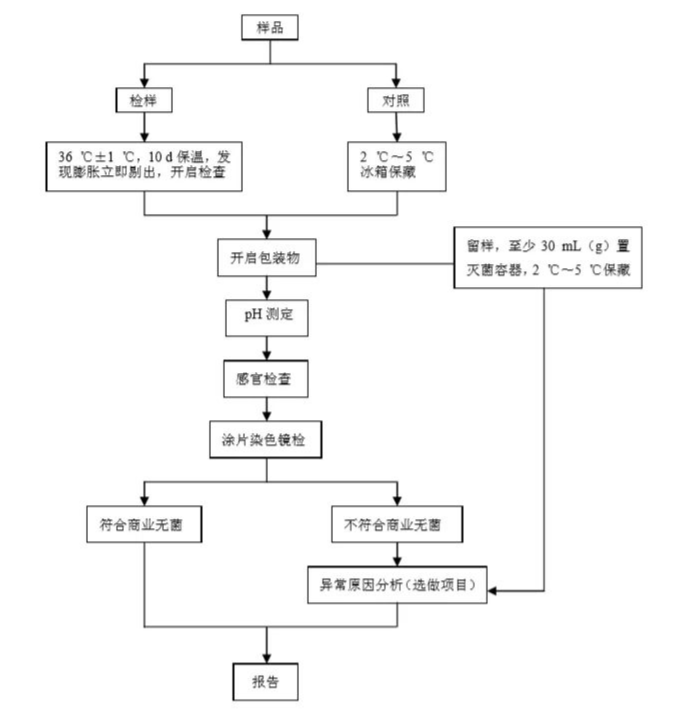
After the sterility inspection procedure shown in the figure below, the sample has not leaked after the insulation test; after the insulation is opened, the sensory inspection, pH measurement, and smear microscopy confirm that there is no microbial proliferation, and the sample can be reported as commercial sterile.
 3. Disinfection
3. Disinfection
Kill or remove pathogenic microorganisms on the media to achieve harmless treatment. Generally speaking, disinfection refers to killing the microorganisms in the environment, preventing the bacteria in the environment from contaminating food again and causing the colony to exceed the standard.
For example, the cleaning and disinfection of food contact surfaces, hand washing and air disinfection, etc., are all targeted at the environment where the product is located.
Disinfection is generally ineffective to microbial spores. Disinfection does not need to kill all the microorganisms in the system. As long as the predetermined requirements are met, it is generally necessary to remove or inactivate the disease-causing and conditional microorganisms in the system.
Disinfection can be divided into high-level disinfection, medium-level disinfection, and low-level disinfection.
Common disinfection methods in food workshops include:
(1) Disinfection method: It can kill pathogenic microorganisms, including ultraviolet disinfection, ozone disinfection, chlorine-containing disinfectant, etc., such as: UV disinfection and ozone disinfection of the air in the workshop; disinfection of water sources, sodium hypochlorite disinfection and soaking work clothes.
(2) Moderate disinfection method: It can kill pathogenic microorganisms other than spores, including heat disinfection, alcohols, phenols, ultrasonic waves, etc., such as skin disinfection with 75% alcohol, boiling at 70-75°C for 30 minutes or Boil at 80℃ for 15min, 100℃ for 5-6min, etc.
Treatment to kill or remove microorganisms on the media, including bacterial spores with strong resistance.
Note that sterilization is for microorganisms, including not only bacteria, but also spores, viruses, fungi, mycoplasma, chlamydia and so on.
The methods that fall into this category include: thermal sterilization (steam, dry heat), ultra-high pressure sterilization, ionizing radiation sterilization (≥25kGy), microwave sterilization, plasma sterilization and other physical sterilization methods, as well as the use of oxyacetic acid, A method of sterilization by chemical methods such as hydrogen peroxide.
Sterilization effect evaluation commonly used biological indicator bacteria: Bacillus Stearothermophilus (Bacillus Stearothermophilus ATCC 7953 or SSI K31) spores.
The amount of recovered bacteria is 5×105cfu/tablet~5×106cfu/tablet, or 5×105cfu/ml~5×106cfu/ml.
The survival probability (ie sterility assurance level, SAL) of microorganisms in terminally sterilized products shall not be higher than 10-6.
High temperature sterilized milk (UHT milk) is an instantaneous sterilization process of ultra-high temperature instantaneous sterilization (135°C to 150°C, 4 to 15 seconds) to completely destroy the microorganisms and spores that can grow in it.

2. Sterile
Sterility is the result of sterilization, meaning that it is completely free of microorganisms. The operation technique to prevent microorganisms from entering the body or object becomes aseptic operation.
However, due to the limitations of the current testing methods, the concept of sterility cannot be applied to the evaluation of the sterility of the entire batch of products. Therefore, the concept of "sterile" currently used is "sterile" in the sense of probability.
After terminal sterilization, the sterility assurance level of sterile products is that the probability of residual microbial contamination ≤ 10-6.
"Commercial sterility" refers to the state in which canned food undergoes moderate heat sterilization and does not contain pathogenic microorganisms or non-pathogenic microorganisms that can multiply in it at normal temperature (GB8950).
After the sterility inspection procedure shown in the figure below, the sample has not leaked after the insulation test; after the insulation is opened, the sensory inspection, pH measurement, and smear microscopy confirm that there is no microbial proliferation, and the sample can be reported as commercial sterile.

Kill or remove pathogenic microorganisms on the media to achieve harmless treatment. Generally speaking, disinfection refers to killing the microorganisms in the environment, preventing the bacteria in the environment from contaminating food again and causing the colony to exceed the standard.
For example, the cleaning and disinfection of food contact surfaces, hand washing and air disinfection, etc., are all targeted at the environment where the product is located.
Disinfection is generally ineffective to microbial spores. Disinfection does not need to kill all the microorganisms in the system. As long as the predetermined requirements are met, it is generally necessary to remove or inactivate the disease-causing and conditional microorganisms in the system.
Disinfection can be divided into high-level disinfection, medium-level disinfection, and low-level disinfection.
Common disinfection methods in food workshops include:
(1) Disinfection method: It can kill pathogenic microorganisms, including ultraviolet disinfection, ozone disinfection, chlorine-containing disinfectant, etc., such as: UV disinfection and ozone disinfection of the air in the workshop; disinfection of water sources, sodium hypochlorite disinfection and soaking work clothes.
(2) Moderate disinfection method: It can kill pathogenic microorganisms other than spores, including heat disinfection, alcohols, phenols, ultrasonic waves, etc., such as skin disinfection with 75% alcohol, boiling at 70-75°C for 30 minutes or Boil at 80℃ for 15min, 100℃ for 5-6min, etc.
(3) Low-efficiency disinfection method: it can kill bacterial propagules and lipophilic viruses, including single-chain quaternary ammonium salts, biguanides, etc.